(Unique Characteristics of Quantum Particles)
1. Wave-Particle Duality
What It Means:
Quantum particles (like electrons or photons) can behave like particles and waves depending on how we observe them.
Example: Double-Slit Experiment
- If we shoot photons (particles of light) through two slits onto a screen, they create an interference pattern, like waves do.
- But if we try to observe which slit each photon goes through, they act like particles, and the wave pattern disappears.
This shows that quantum particles don’t strictly behave like traditional particles or waves—they can act like both depending on the situation.
2. Superposition
What It Means:
A quantum particle can exist in multiple states at once until it is measured or observed. After measurement, it “collapses” into one of those possible states.
Example: Schrödinger’s Cat (Thought Experiment)
- A cat in a box is both alive and dead (superposition) until someone opens the box and checks.
- Similarly, an electron can be in multiple energy levels at the same time until measured.
📌 In quantum computers, qubits use superposition to represent both 0 and 1 at once, giving them vast processing power
3. Entanglement
What It Means:
Two or more quantum particles can become entangled, meaning their properties are linked even across large distances. If you measure one particle, you instantly know the state of the other, no matter how far apart they are.
Example:
- Two entangled electrons are created.
- If one electron is measured to have spin up, the other must have spin down, even if it’s on the other side of the galaxy.
This phenomenon, which Einstein called “spooky action at a distance,” has been experimentally confirmed and is the basis for quantum teleportation and quantum cryptography.
4. Quantization
What It Means:
Physical properties like energy, angular momentum, and charge only exist in discrete amounts (quanta), not continuous values.
Example: Electron Energy Levels in Atoms
- Electrons orbit the nucleus in fixed energy levels.
- They can only jump between levels by absorbing or emitting specific amounts of energy (photons).
- They can’t exist between levels, just like a staircase—you can stand on a step, not between steps.
This explains why atoms emit specific colors of light (spectral lines).
5. Heisenberg’s Uncertainty Principle
What It Means:
It is impossible to know both the position and momentum (or velocity) of a quantum particle with perfect accuracy at the same time.
Formula:
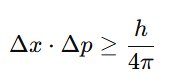
Where:
- Δx = uncertainty in position
- Δp= uncertainty in momentum
- h = Planck’s constant
Example:
- If you try to measure an electron’s exact location, you lose precision in knowing its speed—and vice versa.
This isn’t due to limitations in instruments—it’s a fundamental property of nature at the quantum level.
6. Probability and Wave Functions
What It Means:
Quantum particles are described by a wave function (ψ), which contains all the information about the system. The wave function doesn’t give exact answers, but probabilities of where a particle might be found or what its properties are.
Example:
- An electron in an atom doesn’t orbit like a planet. Instead, it exists in a “cloud” of probability.
- Where it is most likely to be found is determined by the square of the wave function:
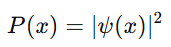
In quantum mechanics, we deal with probabilities, not certainties. This is a major departure from classical physics.
Summary Table
| Principle | Description | Example |
|---|---|---|
| Wave-Particle Duality | Particles act like waves and particles | Double-slit experiment |
| Superposition | Particle can be in multiple states until measured | Schrödinger’s cat, quantum computing |
| Entanglement | Linked particles share states, even at great distances | Quantum teleportation, spin pairs |
| Quantization | Energy and other properties exist in fixed values | Electron orbits in atoms |
| Uncertainty Principle | Cannot precisely know both position and momentum | Measuring electron position affects momentum |
| Probability & Wave Function | Describes likelihood of a particle’s location or state | Electron cloud in an atom |